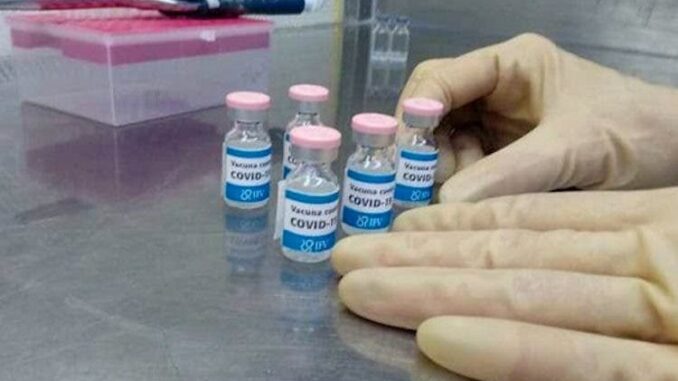
Cuba began a new clinical trial aimed at curing the COVID-19 disease, produced by the SARS-CoV-2 virus, which to date has caused more than 40 million infections in the world.
This new “vaccine candidate” was registered by the Finlay Vaccine Institute under the name of SOBERANA 01A, which today will start being applied in two or three doses.
Through its Twitter, the Institute announced last night that it had registered a new clinical trial in the public registry to evaluate different formulations and dose schedules. The best will advance to higher phases of clinical trials.
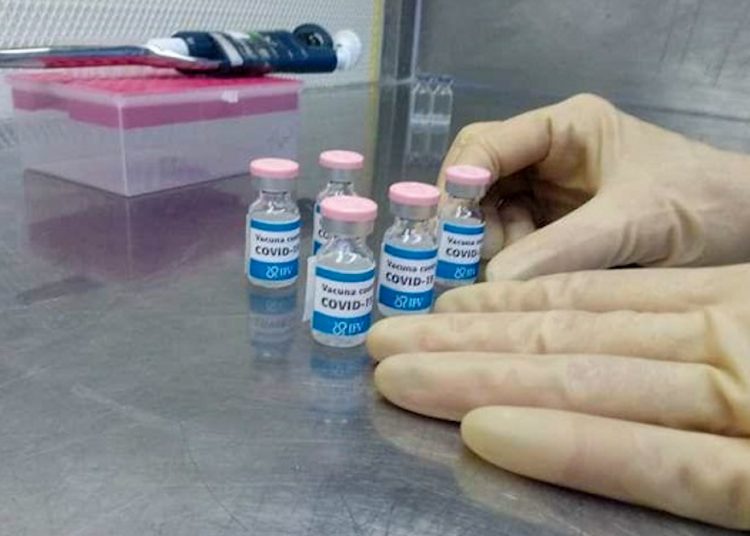
Phase I of the study would take place between October 19 and November 9, the completion of which is scheduled for February 2021.
The details of the new vaccine can be consulted in the Public Registry of Clinical Trials, where SOBERANA 01, the first Cuban coronavirus vaccine candidate to begin clinical trials in humans, has been registered since August.
SOBERANA 01 is in phases I and II of clinical trials with just over 700 volunteers. Its trial period has elapsed without adverse effects, according to what has been reported.
Today, October 19, the World Health Organization (WHO) has registered 44 vaccine candidates in clinical trials, including SOBERANA 01.

Leave a Reply